Arbour Group’s Pharma 4.0 Insights
Thursday March 31, 2022
To understand the meaning and mission behind Pharma 4.0, it is essential to learn more about Industry 4.0. Industry 4.0, also described as the fourth industrial revolution, takes the past emphasis on digitalization to its limits through automation, interconnectivity, machine learning, and real-time data. In a sense, Industry 4.0 connects the digital to the physical, creating a level of cohesion and visibility unattainable before now. Industry 4.0 offers a comprehensive and interlinked approach to manufacturing and allows for better collaboration and access across vendors, partners, and departments through the strategies organizations have adopted.
Given the uncertainty of regulations surrounding advancements not fully realized, the pharmaceutical and broader life sciences industries have historically been slow and cautious to adopt emerging technologies. Pharma 4.0, a term recently conceived by the International Society for Pharmaceutical Engineering (ISPE), is the mission to bring the progress and technologies of Industry 4.0 to the pharmaceutical industry to deliver accessible, cost-effective, high-quality treatments to patients.
The Bumps in the Road to Pharma 4.0
The main challenges organizations in the pharmaceutical industry currently face are interoperability between machinery and systems, the complexity of technical integration, and the lack of harmonized standards. With the expansion in compliance standards, evolving validation, and data integrity requirements, life science organizations are finding themselves in a situation where it is increasingly challenging to keep up with their GxP regulatory commitments. Reliance on legacy systems, growing cyber security risks, and a digital skills gap in AI, IoT, and other emerging technologies make adopting new technologies increasingly tricky within the pharmaceutical industry. These sticking points need addressing to enable pharmaceutical plants to realize the benefits and value this revolution can bring. In addition to the lack of digital maturity, there are few blueprints to follow for this shift to digitization.
Let’s Create a Blueprint
Over the last 3-4 years, the volume control for industry collaboration on digitization and technology of the future has steadily turned up. The past two years have seen significant shifts in that vision in response to external factors. Collaboration realizes the revolution needs to happen across the industry rather than at a company level. By collaborating and sharing business acumen and technological expertise to fill the skills gaps, organizations can share the risks, produce digital twin proofs-of-concept, accelerate innovation, and establish a digital technologies roadmap for the life sciences industry to follow.
More than Good Wi-Fi
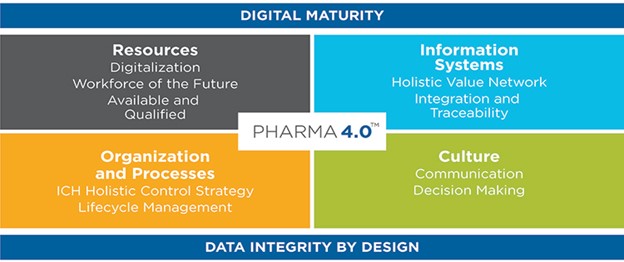
Pharma 4.0 is more than just technological advancements. Many organizations believe the evolution to Pharma 4.0 starts and ends in the IT department, but that is far from the truth. It requires long-term financial and organizational investment, cultural change, and strategic vision to guide an organization’s digitalization roadmap. As this ISPE operating model graphic shows, the areas of your organization that will see the most investment are your Resources, Information Systems, Processes, and Culture. Pharma 4.0 takes these integral components of your organization and creates an unmatched comprehensive operating model compared to old industry standards.
From Industry 4.0 to Pharma 4.0™ Operating Model, ISPE
Who Will Benefit?
Pharma 4.0 allows for adaptive process control, real-time decision-making, and predictive problem solving, allowing for improvements and changes to the product life cycle.
Pharma 4.0 encapsulates the impact that it will have on pharmaceutical manufacturing. Some of the main changes to look for in the future are the vertical networking of intelligent production systems (“smart factories”), disruptive technologies, and new standards of patient-focused treatment delivery made possible through personalized medicines and Just-In-Time projects. With the increase in demand for innovative technologies, we will begin to see more systems and machinery designed to be “smart” that will self-diagnose wear and tear, allow inter-system communication with one another in real-time, and make automatic changes. This will be paired with technologies thought to be out of reach based on costs, such as 3D printing, AI, robotics, drones, AR, nanotech, etc.
Powered by cloud computing, organizations will benefit from efficient sharing and analysis of vast amounts of information (big data), continuous improvement of productivity and supply chains, fewer exceptions with Right First Time quality, and lower costs that ultimately lead to competitiveness and better profit margins. The patient will also benefit from a higher standard of care and new innovative products developed through these connected processes.
The Arbour Advantage
The challenges of technological integration and change can be daunting for any business looking to move towards a Pharma 4.0 strategy. Arbour Group can help mitigate the challenges brought on by Pharma 4.0 and help your organization realize its full potential through our regulatory expertise and proven processes. If you would like to learn more about how Arbour Group collaborates with life science organizations to address the challenges of emerging technologies, contact us today.